Autores: Pablo G. Lustemberg, Chengwu Yang, Yuemin Wang, M. Veronica Ganduglia-Pirovano, Christof Wöll
Artículo.
J. Am. Chem. Soc. 2025
Fecha: FEBR. 2025.
Doi: 10.1021/jacs.4c17658.
Resumen:
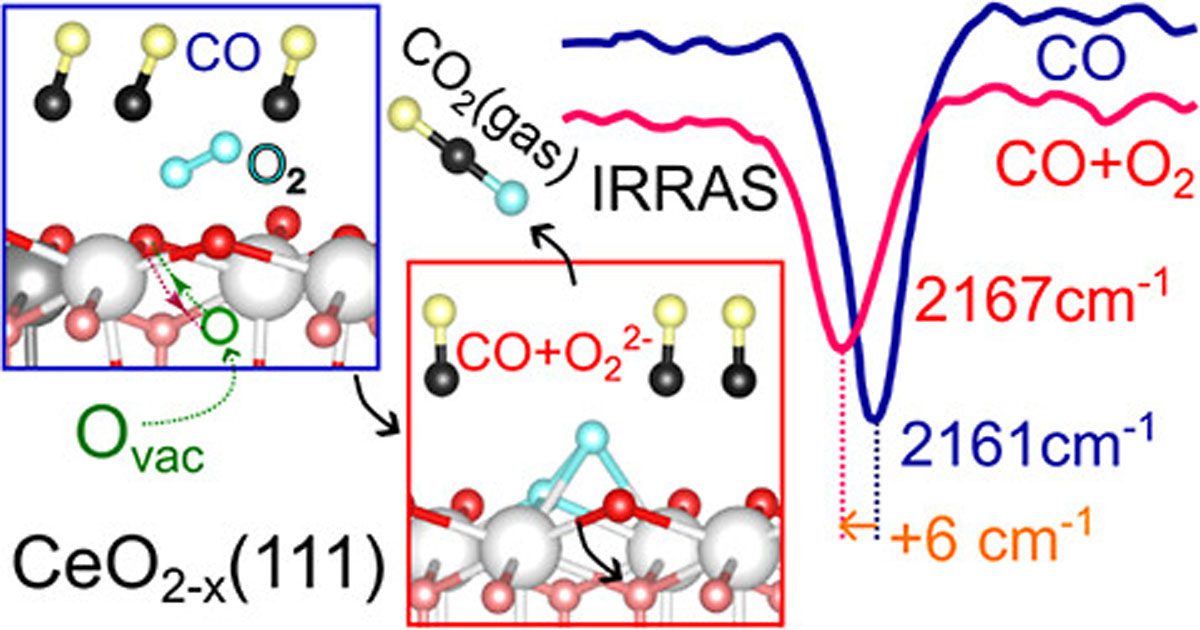
The mechanisms underlying the reaction between carbon monoxide (CO) and activated dioxygen on metal oxide substrates to produce CO2 remain poorly understood, particularly regarding the role of oxygen vacancies and the nature of the activated O2 adsorbate. In this study, we present experimental findings from infrared reflection–absorption spectroscopy on a model system of bulk monocrystalline CeO2(111). Contrary to expectations, exposing the reduced surface to dioxygen (O2) at 80 K does not yield activated oxygen species, such as superoxo or peroxo. Notably, in the presence of adsorbed CO, an unexpected low-temperature oxidation reaction occurs, consuming CO while oxidizing the CeO2 substrate. Since a direct reaction between impinging O2 and adsorbed CO is unlikely at these low temperatures, a novel mechanism is proposed. Extensive spin-polarized density functional theory (DFT) calculations reveal that oxygen vacancies play a critical role in this low-temperature CO oxidation. Initially located in the subsurface region (Vss), these vacancies migrate to the surface (Vs) via a concerted interaction with coadsorbed CO and O2, leading to O2 activation and the formation of superoxo or peroxo species. Detailed analysis identifies key reaction intermediates and quantifies their adsorption energies and activation barriers. Our findings suggest that the peroxo-mediated pathway, with its lower activation barrier, is more favorable for CO oxidation at low temperatures compared to the carbonate pathway. This study provides valuable insights into the dynamic role of subsurface oxygen vacancies in the activation of gaseous O2 and CO oxidation mechanisms on CeO2.